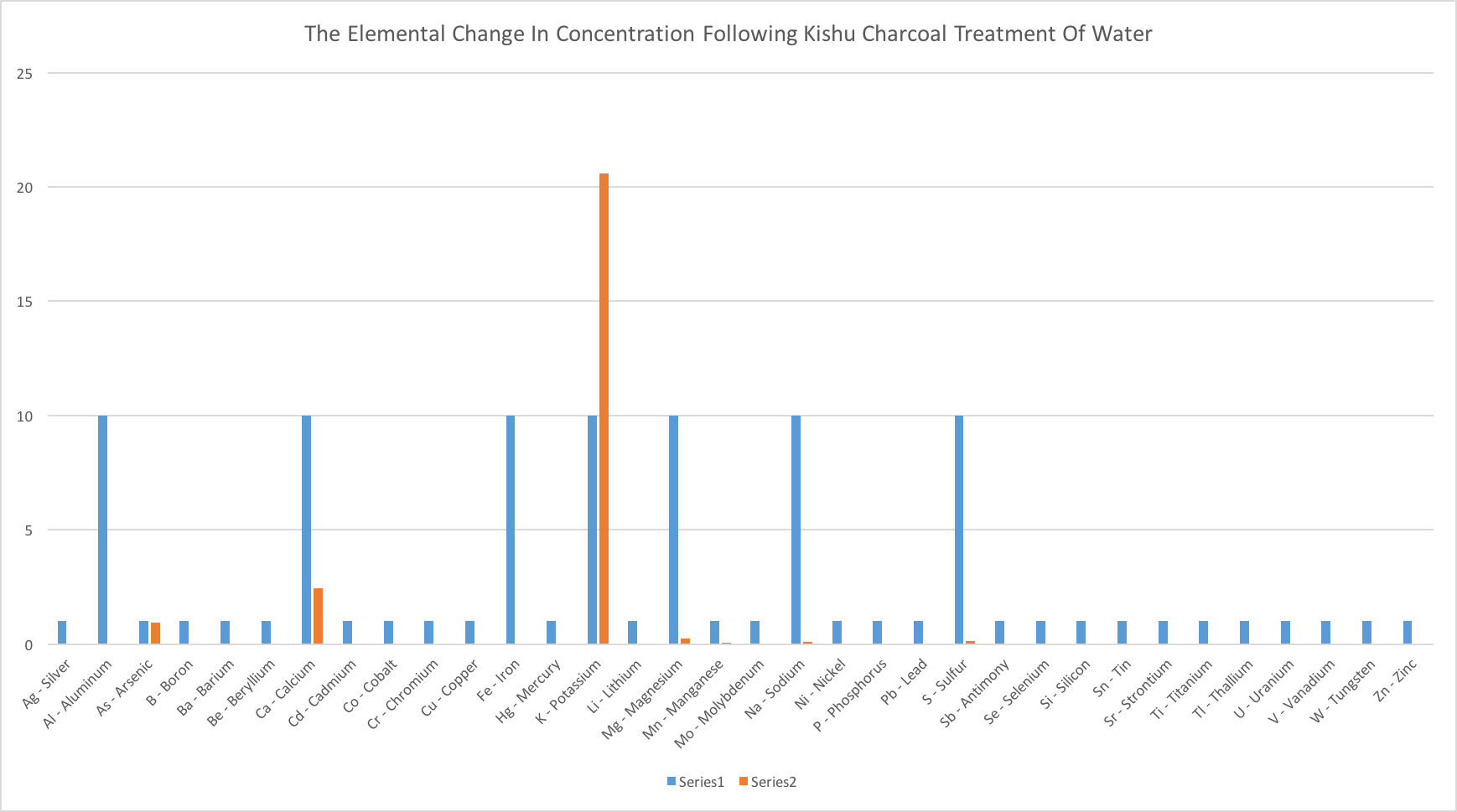
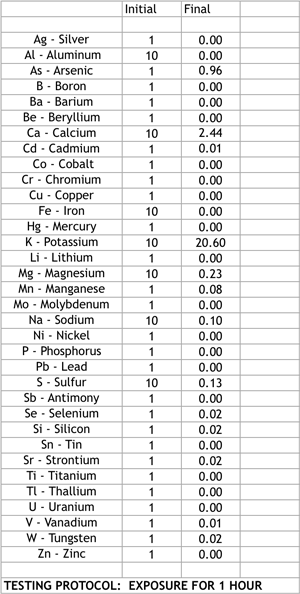
The Science Behind Kishu
This pure form of carbon readily adsorbs or bonds with toxins, principally metals, at the molecular level. Kishu Charcoal has been found to be effective at reducing: LEAD, MERCURY, COPPER, ALUMINUM, URANIUM, and MOLYBDENUM to name a handful of those we tested. In addition, Kishu Charcoal imparts three minerals: Calcium, Magnesium and Potassium.
Although we have not yet conducted the complicated testing required for Chloramines, in my experience, activated carbon and granulated carbon have been used to remove chloramines. However, the water needs to be in contact with the carbon for an extended period of time in order for this removal to be effective. As is already recommended in general for the use of Kishu Charcoal, the Kishu sticks should be in contact with water overnight to achieve the full effect of chloramine removal.
For the complete testing data, please see the detailed chart and graph below.
James R. Self, Ph.D.
Colorado State University
Soil, Water, Plant Testing Lab
ALL MEASUREMENTS ARE PARTS PER MILLION (PPM)